Everything is Done Manually
Ritsuko Morita, our interviewee this time, was introduced to us by Mika Yoshimura (Behind the scenes of cutting-edge research). The interview took place in January 2021, but since she was in the middle of submitting her paper to the journal Nature, we held off on releasing this interview until the paper was published. Now that it’s been published, we can share the interview and reveal the behind-the-scenes work of her paper.(Press release of A telescopic model of the development of hair follicles)
Technique for monitoring deeper layers
I’ve noticed that the images of the area around the root of the hair are 3D movies. How are those movies made?
We take multiple 2D images at different depths and stack those images to create 3D images. When we take those images at regular time intervals, you get something like a flip book, and this is what we call “time-lapse.” This technique where we monitor live organisms or cells in time-lapse is called live imaging.
Some of the images show a deeper layer of the skin. How do you shine the light that deep?
We use fluorescent proteins to label the cells and structures that we are interested in. Then we use a two-photon excitation microscope to detect their fluorescence. As you can infer from the name, it applies two photons worth of energy to the fluorescent proteins to excite and make them fluoresce. Generally, long-wavelength light penetrates the animal body better. If we can use two photons to excite the fluorescent proteins, we can halve the energy of each photon, which in turn allows us to use a longer light wave. The longer the wavelength, the deeper the light can penetrate into an object.
So this is a similar concept to using near infrared radiation for non-destructive inspections of fruits.
Another benefit of using two photons is that it can reduce the damage caused by the light on a sample. This allows us to monitor a sample for a longer time period.
Longer exposure to light can deteriorate the samples?
Yes. We monitor them for a relatively long time.
Do you have any moving images?
Sure.
Process of hair growth
This is the live imaging of the early developmental stages of whisker hair follicles dissected out from a mouse embryo.
Wow. You can see the pores are being formed!
That’s exactly what is being captured. The epithelial tissue invaginates downwards and wraps around the dermal papilla (mesenchyme). Then, this interaction with the dermal papilla causes the epithelial cells to proliferate and differentiate, and in turn, migrate upward and appear as hair on the surface of the skin. The slit from which hair appears is a pore.
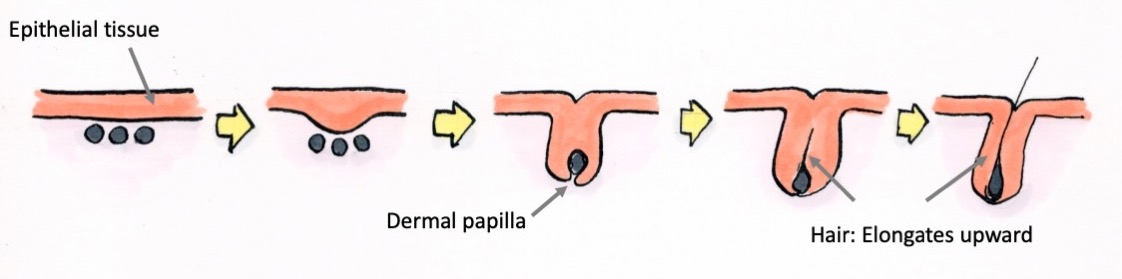
Where do those cells come from?
Cells from the base to the middle region of a pore are undergoing differentiation. Cells can differentiate and transform into other cell types or proliferate to create copies of themselves. Within the hair follicles, there are areas where cells are actively differentiating and other areas where many cells are proliferating. In the proliferative areas, new cells are produced rapidly through cell division, but when those newly produced cells leave these areas, they start to slowly differentiate and eventually become hair.
Until it finally detaches and falls out.
That’s right. The life cycle of hair is to grow, eventually fall out, and then grow again. During this hair cycle, the lower part of the hair follicle which produces hair regenerates over and over again. This repeating cycle is possible because the hair follicle has a population of stem cells capable of generating the constituent cells of the hair follicles. This is a notable characteristic of hair follicle as an organ.
So, something that didn’t exist in the beginning, suddenly appears, grows and transforms… What’s really happening there?
That’s the very theme of our research. In order for organs like hair follicles to function correctly, the stem cells and differentiated cells need to be positioned correctly to work together. We analyzed how hair follicle stem cells and differentiated cells are produced during the fetal stages, by tracking cells in time-lapse images of hair follicle development. When we rewind those time-lapse images, we can find out where those differentiated cells were initially located. Based on that information, we aimed to find the developmental origin of the hair follicle stem cells.
Do the stem cells not stay in the same location?
In the early stages of hair development, the epithelium starts off as a thin sheet, and only parts of this epithelium forms primordial hair follicles (the detailed mechanism has been omitted), which then invaginate, and only some of those epithelial cells become stem cells.
I thought that the stem cells were always present…
I can understand why you’d think that way. Actually, there hasn’t been much analyses done on where the stem cells arise from.
With our live-imaging technique, we can capture the development of hair follicles from the beginning to the end where it produces hair at the single-cell level. If we can track the cells in the time-lapse movies and then rewind the images, we may be able to find where the prospective stem cells originally came from. This was the initial idea of our research.
It had to be done manually
As a person with no training in this field, I’d think that you would be able to automate the tracking of the cells in the images. Was this the case?
While it’s true that automated tracking technology has been advancing, unfortunately it is still difficult to apply it to track the cells in the developing hair follicles that we handle; hence, it’s done manually.
Whoa! Why manually?
It’s because of the sheer number of cells. The cells are also very densely positioned with virtually no space between the nuclei.
When imaging, the nuclei are labeled with a fluorescent protein so that we don’t lose track of them when they divide. However, during early development, cell division is extremely fast and sometimes happens without producing much cytoplasm, leading to cells with the nucleus occupying most of the cell volume. That’s why the images look like the nuclei are tightly packed together.
The nuclei appear circular, but when there are two of them close together, it’s hard to determine if there is one or two.
Unlike human faces, each nucleus doesn’t have a unique feature so it must be hard to distinguish one from another.
Furthermore, sometimes even human eyes can’t tell the difference. If human eyes can’t distinguish them, then it’s difficult for machines to distinguish them as well. Although artificial intelligence and other technologies are actively being developed and used, the technology that can distinguish cells with the quality we need is still not within our reach. That’s why at present it’s basically been done manually.
But if you move the videos back and forth, you can kind of see where and how each cell moved. We use that kind of information to aid our tracking work. It’s the type of work that requires patience and perseverance.
Perseverance for sure.
The truth about the research
How many cells do you track?
The time and duration of tracking are different by images so it’s hard to say, but the total number of cell lineages is probably over 1,200. They divide over the 24 to 72 hours we track them, so by the end of the analyses, it’s probably around 2,500 cells.
Such time consuming work…
Yes. It was a daunting task to do it all myself, so I got help from the technical staff in the lab as well as a college student who came to work part-time for a short-term to the lab. She left saying, “Research really is a repetition of long tedious work.” I guess I was able to convey one hidden truth about research (laughing).
It’s not always flashy and eye-catching.
That’s right (laughing).
ll of that hard work gets summarized into a single diagram…
I think I nearly gave up about five times. I was so anxious about when I would be able to summarize my work into a paper that at one point I announced my resignation, saying, “I quit.”
How do you overcome that defeated mindset?
The simple desire to publish the research that I started. So many people have helped me up to this point, and I knew that I would regret it if I didn’t publish it.
I can understand that. For researchers, if they don’t publish a paper, it’s as if all of their work until that point didn’t happen.
Mysteries of organ diversity
Since we are talking about hair, I’d like to ask you about my kinky hair. How come it doesn’t grow straight?
It’s probably easier to see in the movie, but hair is made up of multiple layers of different cells. When the cells, which differentiate to eventually become hair, move towards the skin surface, they gradually die and become keratinized. They are tightly packed together to become hair.
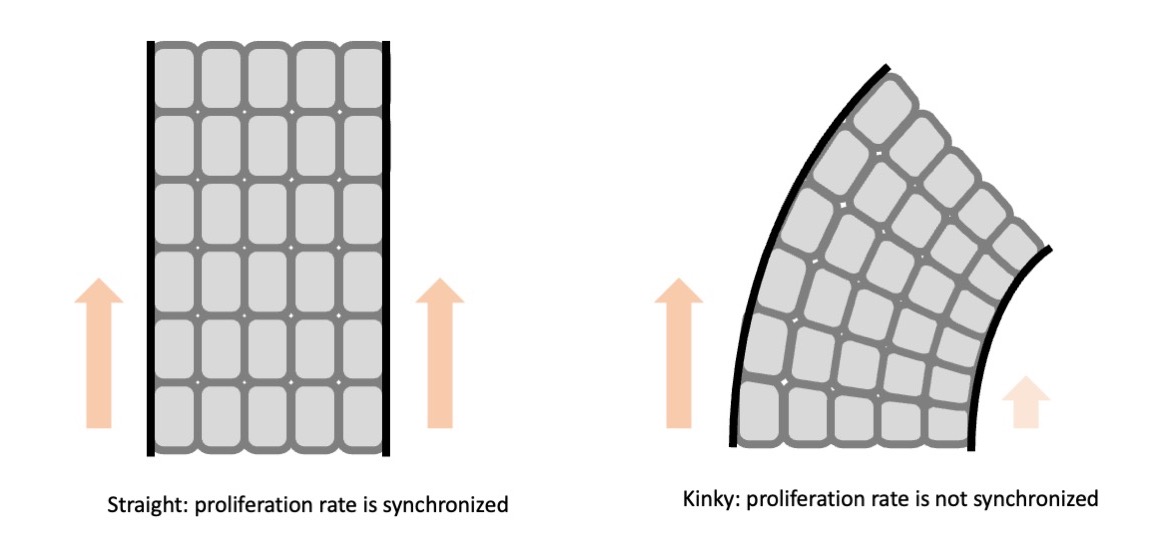
You mean like cuticles?
That’s right. The layers of cells are lined up in a row and move up synchronously. There are about eight layers of cells that move up orderly as they are produced. And the pace at which they move up is in sync. Isn’t that amazing?
If they move up synchronously, the hair grows straight. But if there is even a slight disruption to this pace, distortion occurs which causes kinky hair.
So my hair doesn’t like collective behavior just like me…
Well, it does require a tight teamwork…
Anyway, what are your plans for future research?
In this study, we discovered the developmental origin of epithelial cells and hair follicle stem cells. We also proposed a new model for hair follicle development (not covered in this interview) that explains how the morphogenesis of hair follicles and the correct arrangement of constituent cells within the tissue can occur simultaneously. I’m interested in studying how organ diversity is created through cell-to-cell communications in my next research.
Do you mean that there must be a certain spatial pattern in order for the cells to communicate with each other?
By analyzing the interactions between cells and between tissues, I think that we will be able to clarify the prototype of the patterns that generate morphological diversity, for example instructing to “make a long extension here” or “make a branching here” in organ morphogenesis.
Editor’s Note
Every time I speak to researchers, I am always blown away by the amount of painstaking and time-consuming work that they carry out—from generating thousands of patterns of cells, and developing a system to measure them, to manually counting those cells…
Congratulations on getting published in Nature! Hurray for tedious work!
