A new model organism for lifespan research
This time, I sat down to talk to Dr. Chika Takahashi in the Laboratory for Molecular Biology of Aging, the same lab as Dr. Uno, who I previously interviewed about his research on lifespan using C. elegans. The theme this time also seems to be related to lifespan but I heard that she is using killifish, some sort of tropical fish species. I think I will start by asking, what are killifish?
What are killifish?
I am currently conducting research using the short-lived fish called, killifish.
A short-lived fish. As in they have a short lifespan?
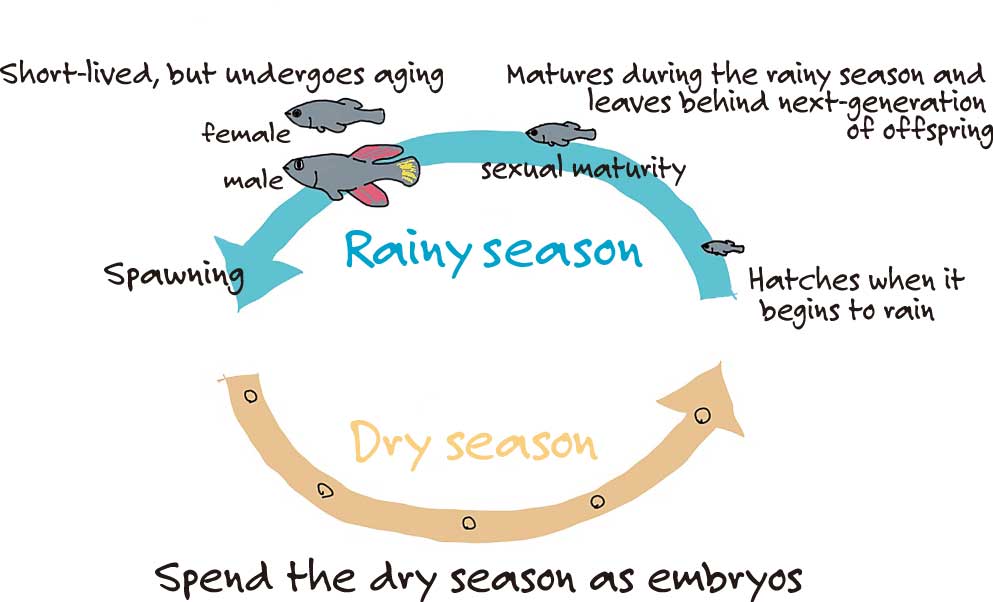
Yes. Killifish are naturally found in southern regions of Africa, such as Zimbabwe, which have dry and rainy seasons. They live in ephemeral ponds that form during the rainy season.

Doesn’t that mean that killifish will die when the dry season arrives?
They lay eggs which are resistant to desiccation. The eggs resist desiccation in the dry season being buried in the soil. As soon as the rainy season arrives, the eggs hatch immediately, and the killifish grow quickly into adults, they spawn, and then die. That is the life cycle of the killifish. Thus, it’s been said that their lifespan became shorter evolutionarily.
I see. That means for the adult stage killifish, they live only for the duration of the rainy season.
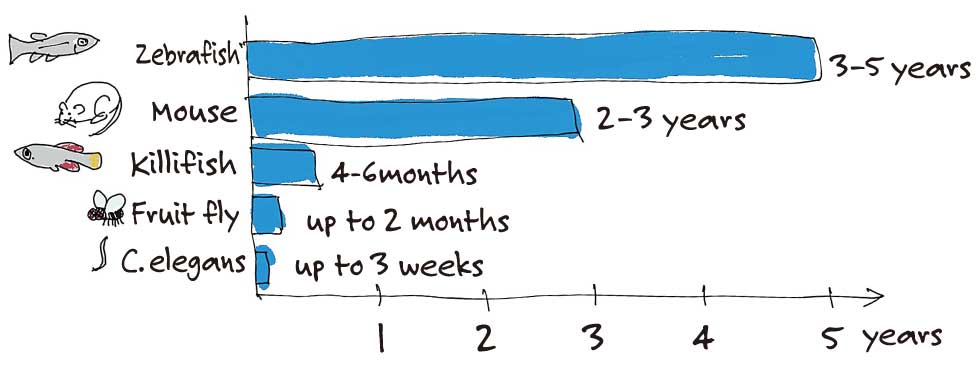
Yes. They also undergo aging processes during that short period of time. Among the vertebrate model organisms that are often used in research today, mice can live between to two to three years and zebrafish can live between to three to five years. In contrast, killifish have a lifespan of four to six months, making them the vertebrate model organism with the shortest lifespan.
I guess insects would be the only other model organism with a short lifespan.
The genome of the killifish was sequenced in 2015, making them a more accessible model organism for aging research.
So, they really are a new model organism.
Yes. Even the protocols for them are still in the process of being established. To begin aging research using killifish, I had to start by setting up my own housing and breeding system and protocol by looking up different reference sources as well as going to visit labs that have already established a rearing system.
Breeding killifish
So, how did you become interested in killifish?
It was following an offer made to me by Dr. Nishida to start up a research project on aging using killifish. Up to that time, I was pursuing research on embryogenesis because I was interested in the processes unfolding from fertilization to body formation, so I was a bit hesitant about accepting the offer. However, I felt both a sense of excitement that I would be able to take on the challenge of conducting interesting research that would take advantage of the short lifespan of killifish and a sense of elation that I would be entrusted with the project from its inception and that I would be working in a different research field to what I had been doing. So I decided to take the plunge and try my hand at working with killifish.
What do you feed them?
In the wild, killifish feed on insect larvae, worms, and zooplankton. But in our lab, I give them baby brine shrimp and bloodworms, which are often used as fish bait. Bloodworms, I buy frozen ones that have been sterilized with ultraviolet light. For the killifish fry, I only feed them baby brine shrimp because their jaws are not yet developed and thus cannot eat bloodworms.
So, how are the eggs handled? I mean the eggs are designed to withstand the inevitable dry conditions to come, right? But in a lab, they would always be submersed in water.
That’s right. So in our lab, we first cultivate the eggs (embryos) in a culturing solution, and upon reaching a developmental stage where they have visible black eyes, the eggs are transferred to humus soil to create a semidry condition and then placed inside an incubator for allow further development.
So, you are creating dry season-like conditions.
When the embryos develop to the pre-hatching stage, we transfer them into a 50 ml tube filled with a humic acid solution, which contains residues of decaying organic matter from humus soil. The tube is then shaken hard to mimic the arrival of the rainy season.
You shake the tube? Is that necessary?
I’ve been using protocols that I have learned directly from other researchers, and I think the intent is to recreate conditions of a sudden rainfall. There are also people who say that the key to the embryos hatching is for them to come into contact with both oxygen and a liquid solution.
How long are the eggs left on the soil until they reach a semidry state?
As short as ten to 14 days. By then the eggs will develop to a stage where they are ready to hatch. After that, depending on the condition of the humus soil, the eggs can be stored in a semidry state for about a month.
How do the embryos survive when they are stored on soil for one month?
They are in a state of dormancy or diapause. In fact, after the eggs are fertilized there are several developmental stages in which the developing embryos can enter a dormant state. Thus, when we say they can live through the dry season in the wild, it means that the killifish have paused their development at a stage where it is possible to enter diapause. When I mentioned earlier that eggs can be left alone for about a month, those embryos are in diapause. Much of the mechanisms of diapause are still unknown.
In the wild, the dry season is about six to eight months long. But when you rear them in the lab, if the period you simulating the dry season is approximately ten days, one would think you could skip the step where you dry them on top of the soil.
There is a reason for not skipping this step. ll at once. But it’s not possible to collect a large number of fertilized eggs in one day because killifish do not lay a lot of eggs at once. We generally use eggs collected over one week, but if you just leave them be after collection, they will hatch on different days because they were fertilized on different days. So we place them in semidry conditions during their developmental phase and the embryos will enter diapause in turn once they reach the pre-hatching stage. When we have collected the number of eggs needed for an experiment, we can make them all hatch at the same time. We’ve also observed under our rearing system that the killifish seem to grow healthier when we include a period where the embryos are dried, hence, why we now include a drying step in our protocol.
Just getting all the systems needed up and running, including your breeding and rearing system, must have taken quite some time.
We began discussions on the design and layout of the breeding room after I joined RIKEN in 2018, and then construction began six months later. The killifish room was finally completed around December 2018. From then on, it was a lot of trial and error to figure out the best breeding and rearing method. And while it did take some time, from the inception of the project, with the helpful advice and cooperation from many, many people, we were finally able to establish our systems. But just as we were getting ready to start our experiments, we were suddenly faced with the coronavirus outbreak, which halted our experiments for about half a year.
Did you save the eggs in a dry state during that time?
We were allowed to continue breeding them to maintain the killifish colony, but we were maintaining only the bare minimum number of killifish.
Can killifish embryos be cryopreserved?
So far there have not been any reports on cryopreservation of embryos or eggs. But there has been a paper published on a method for cryopreserving killifish sperm. According to that paper, their fertilization rate was only about 25 percent, so it does not seem very efficient. In the future, it would be nice to see the development of a more efficient cryopreservation method of sperm or an artificial fertilization technique and maybe even the establishment of a stock center.
Experiments for investigating changes in lifespan
What kind of aging research are you conducting using killifish?
I am currently examining the effects of different environmental conditions during rearing of killifish fry on their growth and development as well as on the subsequent lifespan when they reach adult stages.
Why are you looking at aspects other than aging, such as the killifish fry stages?
Because my past research was looking at processes unfolding during embryogenesis, I decided that if I was going to do aging research with the short-lived killifish, I wanted to focus on the growth and development processes between the killifish fry stage to adulthood, and how that is linked to aging. An advantage of the short lifespan is that it possible to analyze the life stages from the growth and development phases of killifish fry to old age within a shorter period of time. There have also been many reports in recent years of environmental conditions during the pre- and post-natal growth and development being linked to health and disease risks during adulthood.
In our research so far, we are beginning to identify a surprising connection between the growth rate of the fry and lifespan, under certain breeding conditions. In that study, we have been carrying out transcriptome analyses, where we can get a comprehensive look at the expression levels of all genes.
Isn’t it difficult to track gene expression in a single fish?
For fish, a method that is used to track gene expression in a single fish is making a cut in the tail fin of the fish. Younger fish, in particular, can regenerate their tail fins. But in our case, we are looking at gene expression in the whole fish or a specific organ, so instead of tracking one fish, we initially prepare numerous fish and then when they reach a particular age of interest, we collect samples from a few fish at a time.
From time to time, I’ve heard that restricting food intake can result in living longer. Do you see killifish living longer if you give them less food?
They do live longer. There is already a paper published showing this.
In that paper, killifish that were fed twice a day every other day as opposed to being fed normally twice daily showed a ten percent increase in lifespan. There are also reports of other factors besides diet restriction that cause changes to lifespan, with lifespans being extended between 10 to 40 percent, or shortened by about 20 percent.
How do you evaluate whether their lifespan has been extended or shortened?
To determine whether there is a difference in the life curve, we perform statistical analyses such as log-rank tests and other survival analysis tests.
I see. So there are times when the shape of the curve itself changes, and there are other times when the shape doesn’t change much but the timing of the drop in the curve may differ. There are likely also times when the numbers drop dramatically, but the ones that are long-lived will actually survive for a long time.
For that reason, we make sure to perform statistical tests on our data.
Postscript
My first thought was why bother establishing a new animal model, but there is a reason for doing so. Sure, there is a lot that we can glean from C. elegans and fruit flies, but the key point of working with killifish is that they are vertebrates. Compared to killifish, the mice and zebrafish live much longer. And if we were to work on lifespan research using mice, ten years would pass by in no time…
