A physicist contemplating the origins of living things
Dr. Yousuke Ohno, who I previously interviewed about MDGRAPE, had told me that he had been intrigued by a recent Japanese press release suggesting that amino acids could be generated even without nitrogen, so I went straight to the horse’s mouth and talked to Dr. Tomonori Fukuchi who was involved in that study. I was under the impression that he was working on the development of a device that normally can only be used to observe a single nuclide to be able to detect and simultaneously monitor multiple nuclides. So, why suddenly a study on amino acids?
After 5,000 years, half of them will be amino acids?
My interest was peaked by your recent Japanese press release “Amino acids can still be produced without nitrogen—new possibilities for the origin of the amino acids (translation),” hence why I am here to talk to you today.
Thank you very much. I hope you don’t mind if my talk will be something not really biologically oriented…
That’s fine! The press release explained that by using the phenomenon of radioactive decay, it may be possible to make amino acids from compounds that do not contain nitrogen, to which I thought, “Yes, that certainly may be possible.” How did you come up with this idea?
At BDR, I usually work on the development of devices for positron emission tomography (PET). PET is an imaging technology that generates images by using light from the beta particles emitted by radioactive decay of tracers or radionuclides. In addition to PET, I was also developing instruments to detect and measure beta particles directly. I was searching for a good tracer to test this instrument I was developing, and carbon 14 (14C) matched the criteria I had in mind in terms of its availability, half-life, and energy.
I realized that the energy of the beta particles emitted by 14C (decay) is low. If the energy of the beta particles is high, the atom could become dislodged in a process called recoil from the energy produced by beta particle emission. But then, I thought if the recoil energy is low as seen in 14C decay, perhaps the atom won’t become dislodged. In that case, it’s possible that carboxylic acids could become amino acids. Since 14C becomes nitrogen (14N) when it decays, I speculated that it may turn into a new molecule that includes 14N.
Propionic acid includes three carbon atoms linked together and contains a carboxyl group on one side and a methyl group on the other. If the carbon of this methyl group turns into nitrogen to become an amino group, we have an amino acid with a carboxyl group and an amino group̶in this case glycine.
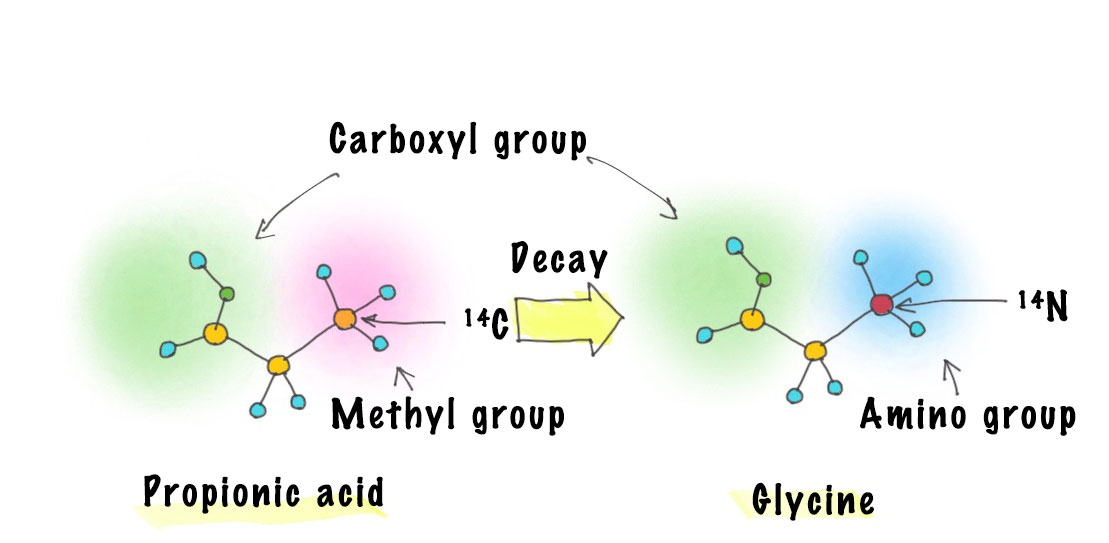
Ah, I see. Nothing will come of it if the carboxyl group becomes separated when 14C decays, but if it remains attached, it’s glycine.
So, I simulated the probability of glycine formation and found that, even if we accounted for some degree of molecular instability, glycine could be produced at least one-third of the time.
That’s a reasonable likelihood! Now that you’ve explained it, I can see what you are saying. That never occurred to me before. There may be many things that can be made in a similar way.
Since this was a simulation, we still need to confirm this experimentally. If you consider the half-life of 14C, it would be ideal to make propionic acid, leave it alone and then check back on it after 5,000 years, but that of course means I can’t confirm it myself (laughing). But the half-life refers to the probability of decay, so if you use the latest analytical equipment to measure trace amounts of the molecule, you should be able to confirm it in a much shorter time frame.
Even if it is a trace amount in a short period of time, when you look at it from the scale of the Earth’s time and physical quantity, it may be sufficiently accumulated. In the field of the origins of life, there are ongoing debates about how amino acids and nucleic acids materialized. Was that something you were interested in?
Actually, I am also working on using PET for fossil imaging, so I did quite a bit of studying around that topic. As a result, I was aware that there is a lot we don’t know about the origins of life.
Another big mystery in relation to amino acids is the existence of optical isomers.
For reasons unknown, life on Earth uses only left-handed or L-amino acids, right? I know there have been many speculations, but there is no definitive theory yet for this either.
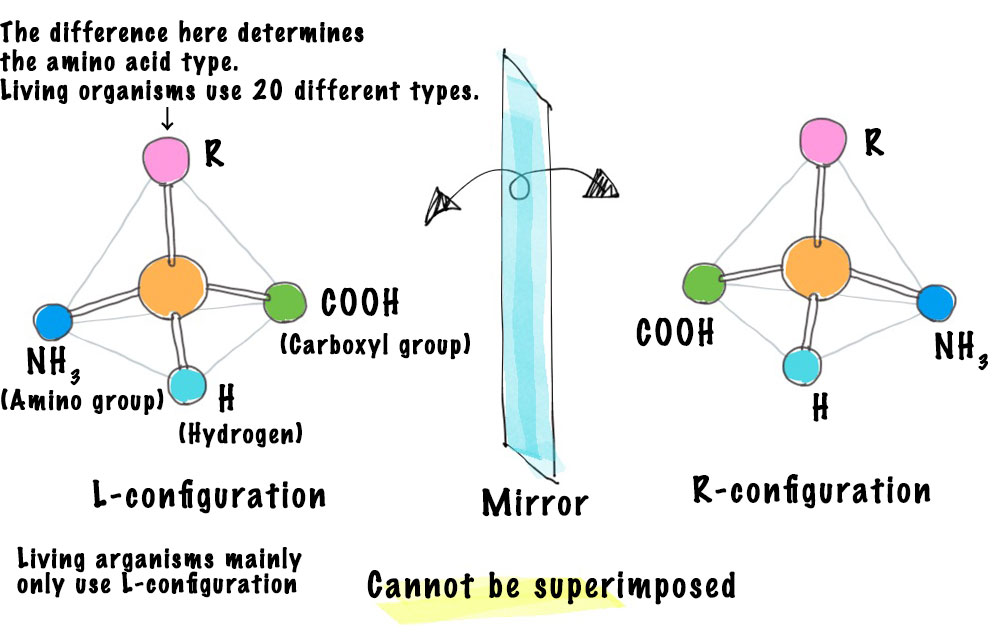
I believe there is a reason for this. In physics, forces are categorized into four types̶gravitational force, electromagnetic force, strong nuclear force and weak nuclear force. Only the weak nuclear force is actually asymmetrical, while the others are symmetrical.
Which makes you think that it might be related to the weak force?
Beta decay, which emits beta particles, is caused by a weak force, so I’m wondering if there might be a connection there. I’ve only just started considering this though.
Development of applications lead to new ideas
You mentioned in passing earlier about fossil imaging, but what do you mean by that?
When developing a device, it’s also important to think about applications of the final product. That is when I came up with the idea of fossil imaging.
Why fossil imaging?
I was vaguely thinking that it would be interesting to measure something that normal people wouldn’t measure, and then I had the opportunity to listen to a talk by an earth and planetary scientist.
It is indeed quite difficult to see inside fossils. Though, lately, we do see stories about CT scans of mummies and such.
That’s right. So, I thought that maybe I would be able to see something with the device I was developing.
How? With PET, you inject a probe with a radionuclide attached to it into the body and detect where it disperses. In the case of cancer diagnosis using PET, you use something like 18FDG (glucose with 18F attached to it), right? But with fossils, it’s impossible to inject something in the first place.
We need to change our thinking and switch the order of how normal PET imaging is done. If we bombard the stable nuclides dispersed in the fossil with a beam of gamma rays from the outside, certain elements will be converted into radionuclides that can then be detected with PET.
I see. So, it works because gamma rays are highly penetrative.
Proton beams are fine as well, but gamma rays can penetrate easier into the rock (i.e. fossil)
So basically, if the stable nuclides became radionuclides that can be detected with PET, they can then be used for imaging. If this is successful, it would mean one would be able to see the internal structures of fossils in three-dimension, right? That would be interesting.
Right? I did a lot of studying while I was considering all the possibilities for my device, and that’s when I came up with the idea of creating the amino acids we were talking about at the beginning.
So that’s the connection.
All roads lead to nuclear physics?
Somehow, I think everything that I have worked on thus far is all connected.
Incidentally, my impression of you was someone working on the development of PET, but what is your background?
Nuclear physics. Particularly, on the experimental side. The number of protons determines what the element is and isotopes of an element have different numbers of neutrons. When the number of protons and neutrons changes, the structure of the atomic nuclei also changes. You may have had an image of the atomic nucleus being roundish, but it can also become oblong.
Whoa, really? I didn’t know that.
I think that you know about the K-shell or L-shell. That is referring to the orbit of electrons, but the atomic nucleus can be understood in a similar way. In the case of electrons, there are eight electrons in the L-shell, right? Well, the atomic nucleus also has similar shell structures, which are determined by how full the shell is. Shells with a good balance of proton and neutron numbers are stable, while those that are imbalanced are prone to decaying. There is also something called magic numbers, and when the proton or neutron numbers reach these numbers, their stability increases even if they are situated in unstable regions. When stable, the shell shape is fairly round, whereas when it is unstable, the shell shape deforms. Likewise, when the atomic nucleus is excited, the shape will change again and the excited state will also have a lifespan.
How do you study the shape and properties of atomic nuclei?
When electrons are excited and then return to the ground state, they emit X-rays; when protons and neutrons are excited and return to the ground state, they emit gamma rays.
Wasn’t there a specific wavelength region for X-rays and gamma rays within the electromagnetic spectrum?
This is often done for convenience when representing them in diagrams, but the original definitions are different. X-rays are emitted from molecules, and gamma rays are emitted from atomic nuclei.
I see. I didn’t know that.
So, I was first doing research measuring those gamma rays to determine the structure of atomic nuclei.
Wow, that story all connected nicely!
Postscript
The name of Center for Biosystems Dynamics Research suggests that it is a research center studying the functions of living systems, but our conversation was primarily centered around physics. But biomolecules are molecules and molecules are made up of atoms, so when you get down to it, I guess it becomes a story around physics and impressed upon me the depth and profoundness of the life sciences as well as the research at BDR.