The Desire to Explain Nutrition Properly
Dr. Chika Takahashi who talked about her research using the short-lived killifish, introduced me to Dr. Hina Kosakamoto, who works with the fruit fly, Drosophila melanogaster. As the name of the lab she works in includes the words ‘nutritional biology,’ I went to the interview hoping that I would be able to learn something that might be useful for dieting.
Changing the food will change the results
You are doing research on nutrition using the fruit fly?
It may come as a surprise, but there is a lot of research on nutrition being carried out with flies and a great deal has been uncovered about the nutrients that are essential for flies. It is estimated that humans consume over 26,000 different nutrients on a regular basis, but flies only require 40 different nutrients. Food specific for fruit flies containing only the minimal number of essential nutrients was developed fairly recently in fact, in 2014.
That’s pretty recent.
Yes. Up until then, each laboratory would make their own fly food from natural ingredients such as yeast, glucose, sucrose, cornmeal and corn flour. But it appeared that experiments yielded different results when food recipes differed, leading to the development of standardized fly food. It’s like the standard cell culture medium.
Do differences in food really change the results?
Yes, it does. In fact, I heard from one of my seniors who is working on hibernation research
using hamsters that he experienced considerable difficulties in reproducing his results when he moved to a different lab and the food fed to the hamsters also changed. In the end he figured out that the vitamin content in the food was different, which led to rather interesting findings. When you move labs, it can be difficult to continue using the same food due to changes in procurement methods and budget-related issues.
In that respect, I guess standardized food is easy to handle. What is the composition of the food?
It contains the 20 amino acids as well as vitamins, minerals, nucleic acids and cholesterol, totaling about 40 different types of nutrients. Oh, and carbohydrates.
That all sounds normal. How are the ratios of the ingredients determined?
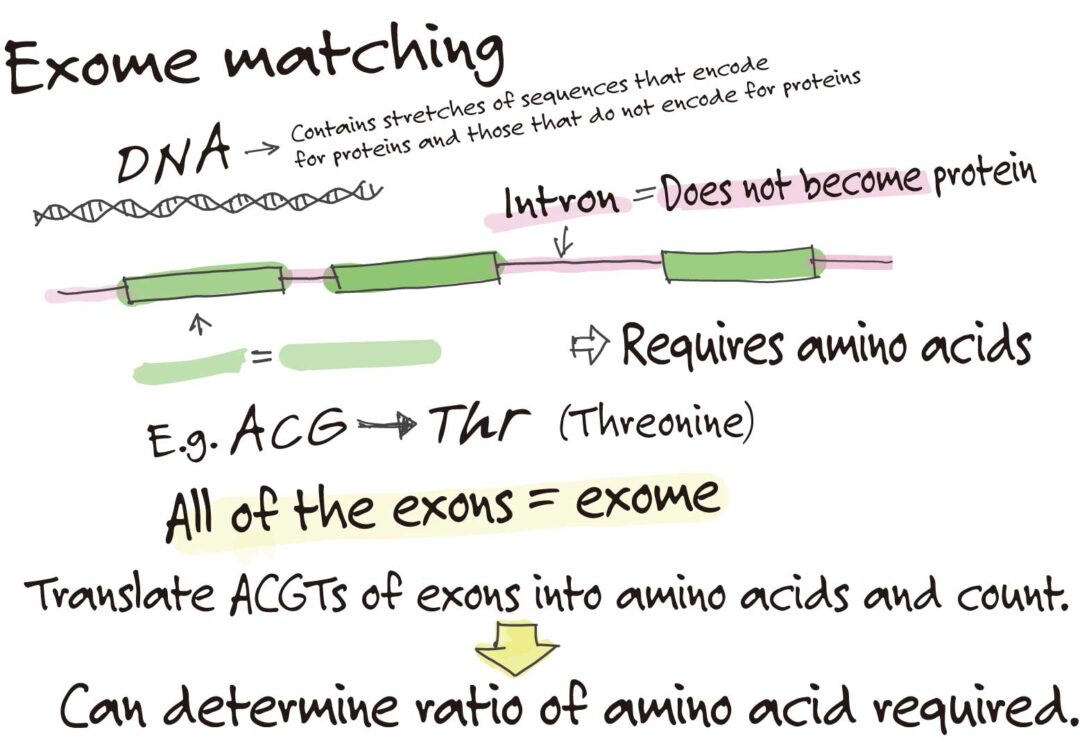
For amino acids, this is determined by a method called exome matching.
Exome matching! I’ve never heard of that.
Exome is a general term referring to all the exons in the genome, the regions that encode genes. Simply put, we determined the ratio of amino acids used to synthesize proteins based on the genetic information encoded in the exons.
Wow, a steamroller approach!
Because we know the composition of this food, it is now possible to remove one nutrient from the food. Thus, it is easier to study the effects of a nutrient on the fly.
A non-essential amino acid is essential for sensing protein scarcity
So you are using that food in your research?
I am using fly larvae in my research and the amount of food they eat changes when their food composition changes. Basically, during the larval stage, the larvae need to grow bigger and will try to consume a lot of proteins. So, they will eat more if the amount of protein in the food is reduced, but the underlying mechanism is not well understood.
So, you are trying to link how the flies sense that they don’t have enough protein and modify their behavior to eat more.
That’s right. I wanted to know what in the food was causing the resulting behavior, so using the food in which I knew exactly what was in it, I tried removing, reducing, or adding each one of the 20 amino acids in the food. This was probably the first time anyone had done this.
You mention so casually that what you did was a world first.
When tyrosine was removed from the food, we discovered that the larvae ate more. By the way, tyrosine is a non-essential amino acid.
What? It’s not an essential amino acid? I thought that we don’t need to eat non-essential amino acids because they can be synthesized by our body. And yet, they can affect behavior?
Interesting, isn’t it? It’s likely that because it is a non-essential amino acid, its importance has never been recognized. From my research, when the amount of tyrosine in the body is reduced by half, it is a sign of starvation.
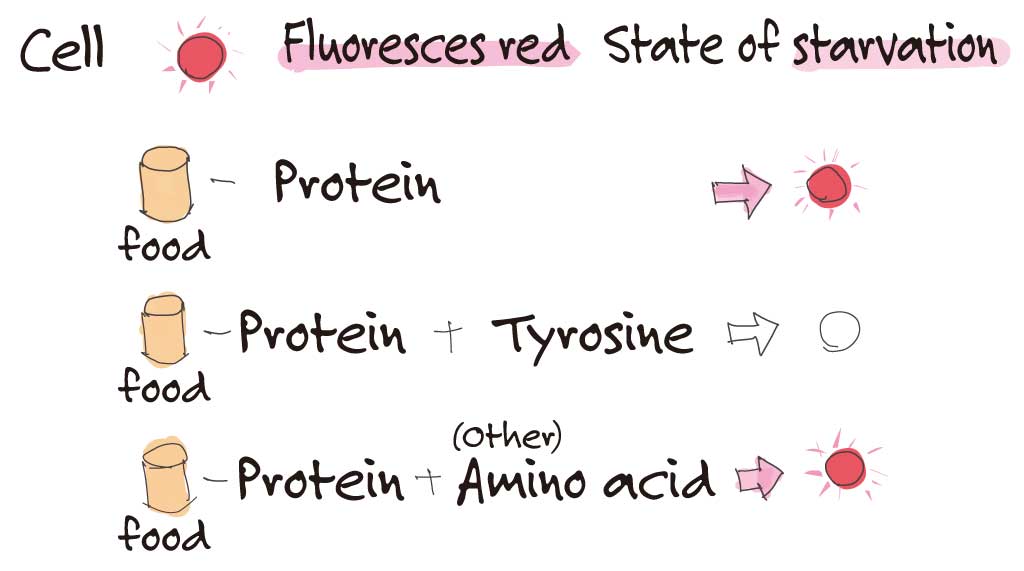
How did you investigate whether or not they were in a state of starvation?
There is a transcription factor that is activated when they enter a state of starvation. We used a genetically modified reporter fly line that will fluoresce red when this factor is activated. This becomes a marker. When protein is restricted, the fly will fluoresce red. If you add one amino acid at a time, sometimes this red fluorescence will disappear.
I see, so that indicates what was lacking in the fly.
That’s right. We also discovered that the phenomenon only occurs in a specific tissue, the fat body.
Detecting shortages
So, being non-essential doesn‘t mean that you do not have to eat it.
The classification of whether an amino acid was essential or non-essential was determined simply by whether or not it was possible to grow without it, and it had not been properly verified whether or not sufficient levels of each amino acid were maintained in the body. There is not much of a difference in the outer appearance of the body when flies do not eat tyrosine. But when we closely examined the amount of tyrosine in the body, we found that the tyrosine had been reduced to half of normal levels.
If it is non-essential, why not just increase the amount synthesized in the body?
The synthesis system does not appear to be a sophisticated one and thus, even if the amount of tyrosine intake is reduced, tyrosine synthesis does not increase. On the other hand, there is also a regulatory mechanism involved in tyrosine breakdown which is tightly controlled. But even with this mechanism, the level of tyrosine in the body is insufficient, and requires increased dietary intake of tyrosine or the body shifts to an energy-conserving state by slowing down processes that require a lot of energy.
How does the body sense that levels of tyrosine are insufficient?
I think there is something like a tyrosine sensor, which I would like to investigate further, but it is extremely difficult. A method for analyzing protein-protein binding has been established, but we haven’t yet fully established a technique for extracting proteins that are bound to metabolites. In this case, it is particularly challenging as the it is bound to amino acids, which are quite small.
Do you have a strategy in mind?
I can think of a few techniques. A simple way would be to coat beads with metabolites and collect the proteins that attach to these metabolites. Or, since the stability of proteins that are attached to metabolites can easily change, we can look at the changes in stability when heat is applied to proteins on its own and those that are attached to metabolites. I believe there’s a way, so I’d like to keep at it a bit longer.
Incidentally, are essential amino acids the same in flies and humans?
They are basically the same. They have been conserved from an ancient ancestor, and in fact, they are also the same for namatode. Our bodies do not need to synthesize those amino acids we can intake from our diet, so this synthetic pathway was discarded a long time ago and it has remained the same since then.
It started with the question “Is that true?”
Why did you decide to study nutrition?
I really love to cook, and there was a period where I spent a lot of time reading recipe books, which naturally led me to becoming interested in nutrition.
So that’s where it started.
Around that time, about 2017, there was a health craze with many TV programs touting things like, “Eat this and you will be healthy.” But when I watched those programs, I often thought, “Is that really true?” It’s not as if a single ingredient can do something to improve health, and even if it could, the mechanism of how it improved health was presented very simply. I began this research because I wanted to be able to explain the mechanism of action at the molecular level.
That’s a pretty clear-cut goal.
At the beginning of my master’s program, however, my research environment was not suitable for nutrition research, so I was doing research on development. It was only in the second half of my master’s program that my research environment was finally favorable for nutrition research, so I changed my theme midway through.
The master’s program is two years, right? Isn’t it difficult to change your research theme with only one year left?
Yes, it is. My advisor told me that if I was going to change my theme at that point, I should do so with the intent of going on to pursue a doctoral degree. That was when I made up my mind—if I am going to get a PhD, I am going to become a researcher.
Postscript
The fly that was mentioned in this interview is Drosophila melanogaster, an organism widely used in life science research. It is a type of fruit fly that on occasion can be spotted flying around your kitchen. When they are well nourished, they can on average lay two eggs per hour and these eggs will develop to adult stages in 10 days, and those adults will then start laying eggs. Learning this fact has renewed my determination to regularly clean up messes.